Welcome to the Cabreiro Lab!
The research focus of our lab revolves around deciphering the molecular intricacies underpinning metabolic disease, cancer, and aging, recognizing their significant medical, social, and economic ramifications. Of particular interest is the burgeoning field of host-microbiota interactions, especially their impact on conditions like obesity, type-2 diabetes, and aging. Despite recent insights into how gut microbiota influence these syndromes, the regulatory mechanisms governing these interactions remain largely unexplored. Distinguishing causality in microbiota-related pathologies presents a formidable challenge, impeding our ability to precisely delineate their role in human health.
To address these gaps, our lab employs a multifaceted approach utilizing model organisms such as C. elegans and rodents. By leveraging these models, we aim to unravel the complex interplay between microbial communities and key physiological processes related to metabolism and longevity. Additionally, our research delves into the influence of microbiota on drug responses in metabolic disease, cancer, and aging, with the goal of optimizing therapeutic interventions and minimizing adverse effects.
Ultimately, our research endeavors are poised to significantly advance our understanding of host-microbiota interactions and their implications for human health. By elucidating the intricate mechanisms through which microbial communities shape physiological outcomes, we aim to pave the way for innovative therapeutic strategies aimed at mitigating the burden of metabolic disease, cancer, and aging on society.
The research focus of our lab revolves around deciphering the molecular intricacies underpinning metabolic disease, cancer, and aging, recognizing their significant medical, social, and economic ramifications. Of particular interest is the burgeoning field of host-microbiota interactions, especially their impact on conditions like obesity, type-2 diabetes, and aging. Despite recent insights into how gut microbiota influence these syndromes, the regulatory mechanisms governing these interactions remain largely unexplored. Distinguishing causality in microbiota-related pathologies presents a formidable challenge, impeding our ability to precisely delineate their role in human health.
To address these gaps, our lab employs a multifaceted approach utilizing model organisms such as C. elegans and rodents. By leveraging these models, we aim to unravel the complex interplay between microbial communities and key physiological processes related to metabolism and longevity. Additionally, our research delves into the influence of microbiota on drug responses in metabolic disease, cancer, and aging, with the goal of optimizing therapeutic interventions and minimizing adverse effects.
Ultimately, our research endeavors are poised to significantly advance our understanding of host-microbiota interactions and their implications for human health. By elucidating the intricate mechanisms through which microbial communities shape physiological outcomes, we aim to pave the way for innovative therapeutic strategies aimed at mitigating the burden of metabolic disease, cancer, and aging on society.
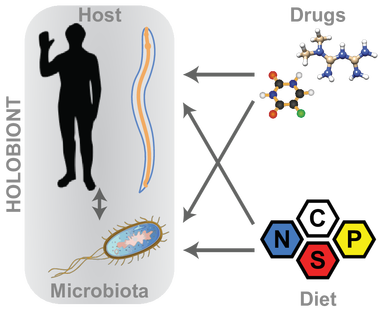
In the natural world we are surrounded by multiple bacterial communities living inside and outside our bodies. Throughout time we have evolved together and established a tight interaction with our microbes. The holobiont - a combined metabolic entity made up of the host and its microbiota is a coherent functioning unit in health and disease and shaped by multiple factors, including diet and exposure to drugs.
Drug-Microbe-Host metabolic cross-talk for healthy ageing
Ageing is one of the major challenges of modern society. Finding strategies to improve lifespan with a concomitant increase in healthspan are critical for developing means to reduce the harmful outcomes of ageing. Pharmacological interventions that target signaling pathways can improve lifespan and healthspan in model organisms, implying that the ageing process can be a point of intervention to achieve broad spectrum protection against age-associated decline. To solve the biology of ageing, we need to understand the physiology of the holobiont: the host and its associated microbes. In particular, how microbes and the host communicate when challenged with exogenous compounds (e.g. diet, drugs) to produce such complex phenotypes. Here we focus on developing a complementary pharmacological pipeline with the potential to unravel drug-diet-microbe-host interactions. For this purpose, we will utilise two tractable genetic models: the bacterium E. coli and the nematode C. elegans, widely used for ageing research. We will combine classical and advanced microbial genetics and high-throughput genomic/chemical approaches with targeted metabolomics at the holobiont level. This strategy will identify drugable mechanisms in bacteria (e.g. signalling/biochemical pathways) that alter microbial metabolite availability with the capacity to regulate host ageing.
Metformin and the Microbiome
Metformin is the most widely prescribed drug to treat type-2 diabetes. However, its therapeutic potential goes far beyond its anti-hyperglycaemic action, also reducing the risk of cancer and ageing. Recently we showed that metformin can modulate the microbiota of C. elegans to induce a dietary-restriction state and extend lifespan. Despite significant research into the functions and the molecular mechanism of metformin, little is known about its specific cellular target(s), in particular those underlying the long-term wide-range effects on mammalian health independent of glycaemic control. The aim of our lab is to address this omission by providing the first hypothesis-driven investigation into the regulation of mammalian health by metformin through its effects on gut microbiota. To test this, we utilise germ-free and gnotobiotic rodents and apply high-end metabolomics and transcriptomics techniques to investigate the role of metformin in modulating mammalian gut microbiota.
Co-metabolism of host and microbiota for the improved efficacy of cancer drugs
Fluoropyrimidines are the first-line treatment for colorectal cancer but their efficacy is highly variable among patients. Combining two tractable genetic models, the bacterium E. coli and the nematode C. elegans, we performed high-throughput screens that unravelled the complexity underlying host-microbe-drug interactions. We report that microbes can bolster or suppress the effects of fluoropyrimidines through metabolic drug interconversion involving bacterial vitamin B6, B9 and ribonucleotide metabolism. Also, bacterial deoxynucleotide pools amplify 5-FU-induced autophagy and cell death in host cells. Our findings suggest a two-way bacterial mediation of fluoropyrimidine effects on host metabolism, and may explain the source of inter-individual variability that impacts drug efficacy in humans.. These findings highlight the potential therapeutic power of manipulating intestinal microbiota to ensure host metabolic health and treat disease.
